Research explores landscape of innovative contracts for gene therapies & identifies key barriers & opportunities for future contract development/implementation.
WASHINGTON, DC, UNITED STATES, December 11, 2025 /EINPresswire.com/ -- New research from the National Pharmaceutical Council (NPC) published in PharmacoEconomics supports consistent and equitable patient access to gene therapies.
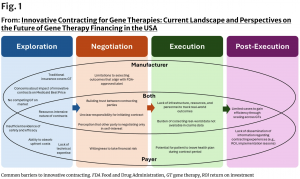
“Innovative Contracting for Gene Therapies: Current Landscape and Perspectives on the
“Gene therapies have the potential to reshape treatment paradigms through one-time, potentially curative interventions. Yet we're trying today to pay for these therapies with yesterday’s reimbursement system,” says Dr. Wagner, NPC Director of Research. “Our landscape analysis of both literature and insights offers a multi-stakeholder view of the challenges and opportunities in gene therapy financing.”
Using a multi-method approach to analyze the existing landscape of publicly available data regarding innovative contracting for gene therapies, the researchers found that innovative contracting existed for 10 out of 14 of the available gene therapies approved by the FDA by January 2025. Additional insights include:
• 50% of publicly available gene therapy contracts involved upfront payments with milestone-based rebates.
• Other commonly included provisions in innovative contracts were upfront payment with warranties and performance-based installment payments.
• Payers may prefer contracts that use both clinical trial outcomes and real-world outcomes to monitor success, contracts for potentially curative therapies (one-time dose) versus multi-dose therapies, and contracts covering a single therapy rather than multiple therapies.
• Manufacturers may be motivated to pursue innovative contracting in order to streamline patient access, mitigate or reduce budget impact, reduce product uncertainties, or fulfill a payer request.
•Barriers to widespread adoption include a lack of mutual trust between payers and manufacturers, a lack of data conveying the return on investment for innovative contracts, a lack of sufficient incentive for stakeholders to engage in contracting, perceived regulatory limitations, and patient portability challenges.
Policymakers are also working to address this issue. The Centers for Medicare & Medicaid Innovation Center (CMMI) launched the CGT Access Model, a multi-year voluntary initiative for state Medicaid programs and manufacturers that initially focuses on increasing access to gene therapies to treat sickle cell disease.
“Innovative contracting has gained traction between payers and manufacturers over the last decade,” says Dr. Campbell, NPC Chief Science Officer. “It will be important that all stakeholders in the gene therapy financing ecosystem — especially patients and their caregivers — play a role in establishing and adopting principles as more of these therapies are made available to patients.”
About the National Pharmaceutical Council
NPC serves patients and society with policy-relevant research on the value of patient access to innovative medicines and the importance of scientific advancement. We envision a world where advances in medicine are accessible to patients, valued by society, and sustainably reimbursed by payers to ensure continued innovation. For more information, visit www.npcnow.org and follow NPC on LinkedIn.

No comments:
Post a Comment